Abstract
Introduction. In rrPMBCL, treatment outcomes with current therapies remain poor. Similar to classical Hodgkin lymphoma, PMBCL frequently exhibits 9p24.1/ PD-L1 / PD-L2 copy number alterations and rearrangements with associated PD-L1 and/or PD-L2 overexpression, which may facilitate immune evasion. The genetics of PMBCL could thus make it particularly susceptible to PD-1 blockade. In the phase 1 KEYNOTE-013 (NCT01953692) trial, the anti-PD-1 antibody pembrolizumab showed promising antitumor activity against rrPMBCL. KEYNOTE-170 (NCT02576990) is an ongoing, two-cohort, multicenter, Phase 2 study evaluating the efficacy and safety of pembrolizumab in patients with rrPMBCL and in patients with Richter syndrome. Here we report updated interim results and biomarker data from the rrPMBCL cohort of KEYNOTE-170.
Methods. This independent cohort of KEYNOTE-170 enrolled patients with rrPMBCL who relapsed after or were ineligible for autologous stem cell transplant (auto SCT); patients ineligible for auto SCT had to have relapsed or refractory disease after ≥ 2 lines of prior therapy. Patients received pembrolizumab 200 mg IV every 3 weeks until disease progression, unacceptable toxicity, or completion of 35 treatment cycles (i.e., 2 years). Tumors were biopsied prior to initiation of therapy and the tissue evaluated for relevant biomarker expression. Treatment response was assessed every 12 weeks by positron emission tomography and computed tomography using IWG 2007 response criteria. The primary end point was objective response rate (ORR) by blinded independent central review (BICR). Key secondary end points were complete response rate, ORR by investigator assessment, duration of response, overall survival (OS), and safety/tolerability. The safety population consisted of all patients who received ≥ 1 dose of study drug and the efficacy population of all safety patients who were expected to have ≥ 24 weeks of follow-up by the analysis cut-off date.
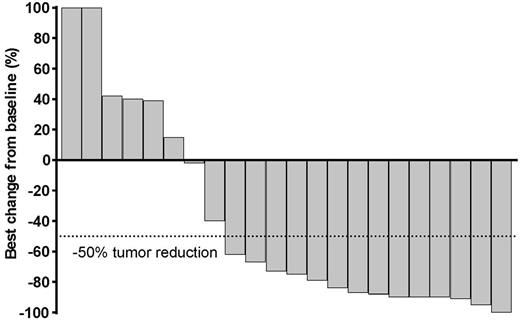
Results. Patients were enrolled at 14 sites in 9 countries. At the analysis cutoff date (14 April 2017), 49 patients were treated in the rrPMBCL cohort (safety population): median age 33 years (range: 20 - 61), 55% female, median 3 lines of prior therapy (range: 2 - 8), 31% with prior radiation, 100% with prior rituximab, and 70% auto SCT ineligible due to chemorefractory disease. In total, 29 patients (59%) were PD-L1 positive, 2 (4%) PD-L1 negative, and 18 (37%) had missing baseline PD-L1 status. Median follow-up duration was 6.6 mos (range: 0.1 - 13.6); 29 patients discontinued treatment due to clinical or radiological progression (n=24), AE (n=3), or physician decision (n=2). In the efficacy population (i.e., patients expected to have reached the 24 week response assessment; n=29), ORR was 41% by BICR and 38% by investigator assessment. By BICR, responses were: 4 complete response (14%), 8 partial response (28%), 3 stable disease (10%), 8 progressive disease (28%), and 6 (21%) no assessment (i.e., discontinued or died prior to the 24 week imaging assessment). Of the patients in the efficacy population, 22 (76%) were positive for PD-L1, 1 (3%) were PD-L1 - negative, and 6 (21%) had missing biomarker data. Among all 22 evaluable patients, 16 (73%) had target lesion reductions (Figure). Median time to response was 2.9 mos (range: 2.4 - 8.5). Median duration of response was not reached (range: 1.1 - 8.2 mos); 10 responses were ongoing (range: 1.9 to 8.2 mos) at data cut-off. Median OS was also not reached; 12-months OS was 62%. In total, 12/49 patients (25%) experienced serious AEs and 26/49 (53%) experienced treatment-related adverse events (TRAEs). Grade 3 TRAEs were neutropenia (n=5 patients), increased hepatic enzymes (n=2), Clostridium difficile infection (n=1), tumor flare (n=1), asthenia (n=1), and pneumonia (n=1). One patient had a grade 4 TRAE (neutropenia). There were no treatment-related deaths. Updated data will be presented at the meeting.
Conclusions. In this ongoing global trial, PD-1 blockade with pembrolizumab showed promising antitumor activity and a manageable safety profile in patients with rrPMBCL, similar to results of the Phase 1b KEYNOTE-013 trial.
Support. Merck & Co., Inc., Kenilworth, New Jersey, United States.
Zinzani: Celgene, Roche, Janssen, Gilead, Takeda, BMS, MSD, Servier, Sandoz, Mundipharma: Honoraria; Celgene, Janssen, Gilead, Roche, Takeda, BMS, MSD, Sandoz, Servier, Mundipharma: Speakers Bureau; Merck: Consultancy, Other: Advisory board. Thieblemont: Bayer: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Walewski: Roche: Consultancy, Honoraria, Other: travel costs, Research Funding; Takeda: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Servier: Consultancy; GSK/Novartis: Research Funding. Fogliatto: Roche: Honoraria, Other: Travel Support; accomodations; Novartis: Honoraria, Other: Travel support; AbbVie: Other: Travel Support. Christian: Pharmacyclics: Research Funding; Merck & Co., Inc.: Research Funding; Seattle-Genetics: Research Funding; Janssen: Research Funding; BMS: Research Funding; Acerta: Research Funding; Genentech: Research Funding; Roche: Research Funding; Celgene: Research Funding; Immunomedics: Research Funding. Özcan: Novartis: Research Funding; Janssen: Research Funding; MSD: Research Funding; BMS: Honoraria, Other: Travel Accomodation; Roche: Honoraria, Other: Travel Accomodation; Abbvie: Other: Travel Accomodation; Takeda: Other: Travel Accomodation; Celgene: Other: travel accomodation, Research Funding; Bayer: Research Funding. Salles: Amgen, BMS, Celgene, Gilead, Janssen, Kite, Merck & Co., Inc., Morphosys, Novartis, Roche, Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding. Shipp: Takeda: Other: Scientific Advisory Board; AstraZeneca: Honoraria; Cell Signaling: Honoraria; Merck: Other: Scientific Advisory Board; Gilead: Other: Scientific Advisory Board; Bayer: Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding. Chatterjee: Merck & Co., Inc.: Employment, Other: stock/stock options. Orlowski: Merck & Co., Inc.: Employment, Other: stock/stock options. Balakumaran: Merck & Co., Inc.: Employment, Other: stock/stock options. Armand: Roche: Research Funding; Infinity: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Sequenta/Adaptive: Research Funding; Tensha: Research Funding; Genmab: Consultancy; Pfizer: Consultancy, Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Affimed: Research Funding; Sigma Tau: Research Funding; Otsuka: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal